The fuel cell: an onboard power-plant
What is a fuel cell? A fuel cell is a device that converts the chemical reaction energy of a supplied fuel and an oxidation agent into electrical energy. Consequently, unlike most fuels that produce energy as the result of a combustion process, hydrogen – the ideal fuel for use in fuel cells – is not “burned” in the cell. Its energy density of 33.3 kWh/kg (15.1 kWh/lb) is nearly three times higher than that of diesel (11.9 kWh/kg; 5.4 kWh/lb). Plus, in a hydrogen-oxygen bond known as water, it is available in practically unlimited supply. After all, 70 percent of the Earth’s surface is covered by water. Moreover, pure hydrogen contains no bound carbon, so the emissions of fuel cells contain no CO2. Fuel cells can operate with all hydrogen-containing gases such as methanol, coal gas and natural gas. However, unlike hydrogen, these gases always emit CO2 as part of the transformation process into electricity and water. The oxygen from the ambient air serves as the oxidation agent.
What are the advantages of fuel cells?
They mainly result from the type of fuel they use: hydrogen. It allows us to store electricity produced from renewable sources by means of electrolysis without harmful emissions and use it as needed. This is particularly important in terms of emissions across the energy chain: from production to fuel tank to wheel. Experts refer to this as well-to-tank and tank-to-wheel or well-to-wheel when looking at the entire chain. Whereas the major losses and emissions of ICE powertrains occur in the vehicle itself, electric powertrains operate with zero emissions, irrespective of whether the traction power is supplied by a battery or a fuel cell. The lion’s share of an electric vehicle’s losses and emissions is incurred during power generation and storage. However, both of these aspects are negligible if the power is produced from renewable energy sources.
How does it work?
A fuel cell transforms the energy bound in a fuel directly into electrical energy. The composition of a low-temperature PEM (proton-exchange membrane) fuel cell – currently the preferred technology – is similar to that of a battery: an electrolyte, in this case a membrane instead of a liquid, separates two plate-shaped electrodes (anode and cathode) – referred to as bipolar plates in the context of fuel cell technology – from each other. This membrane is able to conduct protons but is otherwise impermeable. It is coated with catalytic materials that promote the fission of hydrogen into electrons and protons. The electrons flow as usable electric current to the cathode via a conductor. The protons also flow through the membrane to the cathode where they meet with oxygen ions that have formed as a result of the absorption of the electrons that have migrated to the cathode. Both react into water vapor and wat
What does it look like?
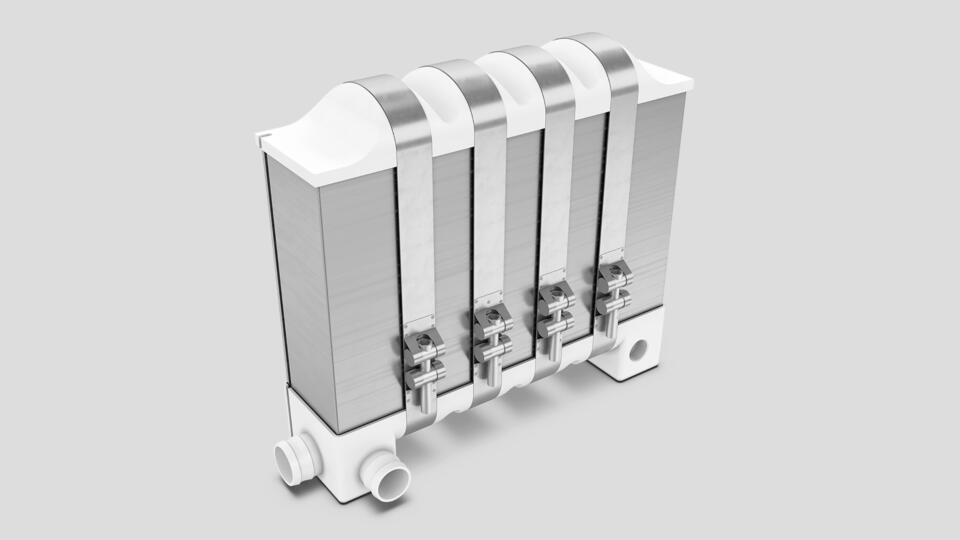
A single cell may be smaller than a matchbox. Modern single cells consist of three flat discs, two of a thin metal and one of a coated polymer film. As the voltage of a single cell is only between 0.5 and 1 volt, several identical cells are combined into a stack. A 100-kW fuel cell system with pumps, lines, heating system and control electronics for a vehicle powertrain fills the engine compartment of a mid-sized car.
Why has it been a niche product so far?
As a combined heat and power producer in buildings it’s (still) too costly. In vehicle applications, the fact that no country has an extensive network of filling stations is another inhibiting factor. It’s the old chicken-and-egg problem: for energy corporations, an expansion of the network is not profitable because there are not enough fuel cell vehicles while the automotive industry argues that there’s a lack of filling stations.
Why is it becoming attractive now?
In the past, there was no need to use them. “After all, we had wood, coal and petroleum. These fuels were ‘lying around’ and just had to be ignited,” explains Michael Fröba, a professor of chemistry at University of Hamburg. However, in view of the pursuit of carbon-neutral, clean mobility to combat climate change hydrogen-operated fuel cells are an intelligent key technology.
Who invented it?
In 1838, the Swiss-German chemist and physicist Christian Friedrich Schönbein immersed two platinum wires in hydrochloric acid together with hydrogen and oxygen and detected electrical voltage between the two wires – the world’s first fuel cell. The British physical scientist Sir William Robert Grove adopted the idea in 1839 and combined several of these cells into a “gas battery” – the world’s first usable fuel cell stack.


Why is a battery still needed as well?
Because fuel cells are unable to recover energy, for instance from braking force, but assistance for powerful acceleration makes sense and because energy must also be available for starting the fuel cell. Such batteries, however, can be much smaller than the traction batteries of battery-electric vehicles (about 10 percent of their size).
How efficient is a fuel cell?
A fuel cell generates electricity and heat. Both electric and thermal efficiency may amount to as much as 65%, depending on the design, without considering losses during production, transportation and storage of the fuel. While the electric power is used for propulsion, the heat can be used for heating the car.
What does the capacity depend on?
Decisive for the capacity and durability of fuel cells are the catalytically coated membrane and materials, as well as the coatings of the bipolar plates. The membrane coatings typically consist of carbon and platinum or a platinum-containing mix as a catalyst. To increase capacity, durability, responsiveness and electric efficiency, manufacturers like automotive and industrial supplier Schaeffler are working on various coatings and electrolytes.

Drei Fragen an …
… Dr. Stefan Gossens, Manager, Innovation Program Energy Storage and Conversion at Schaeffler.
Why is the fuel cell of interest to Schaeffler?
We regard the fuel cell as a viable complement in the field of new powertrain systems and see our expertise in the areas of materials, metal forming, coating and later of course also in mass production. Obviously, the initial focus is on fuel stacks but in a further step, parts of the fuel cell periphery may be relevant, too.
Where does Schaeffler see potential customers?
There’s a wide variety of use cases in mobility, as well as beyond commercial vehicles and passenger cars, in which the emission-free production of electric power is being pursued, such as in the areas of rail, industrial trucks and stationary applications like heat and power cogeneration systems.
In your view, how relevant is the price of a viable fuel cell system to achieving the technology’s large-scale breakthrough? And when will this happen?
Today, the high price of the technology is no doubt still an obstacle to its wide-spread use. The aforementioned industrialization as well as further technological developments will significantly contribute to the necessary price reductions from a mid- to long-term perspective. We’re going to see this happening during the 2020s, in similar ways as we previously did with photovoltaics and lithium-ion batteries.
In future mobility contexts, battery-electric and fuel cell vehicles will be able to ideally complement each other. But the fuel cell will also prove its potential in use cases beyond trucks and passenger cars
Dr. Stefan Gossens
How is hydrogen produced?
By means of the chemical process of electrolysis: A positive and a negative pole are immersed in water which causes the water to split into the H2 and O2 molecules, i. e. hydrogen and oxygen. Specialty coatings applied to the positive pole (cathode) and the negative pole (anode) and the addition of acid to the water accelerate the reaction and increase the yield. The energy invested in electrolysis “is not wasted but partially transferred to the individual molecules where it remains until the reverse reaction occurs,” explains Professor Michael Fröba, PhD.
Are there any maintenance procedures?
Fuel cells are actually deemed to be maintenance-free. Manufacturers typically require an annual inspection, not least due to the other systems such as the vehicle’s brakes and the exhaust system for the fuel cell heater. Their lifecycles are between 5,000 and 40,000 hours, depending on the technology. For a passenger car fuel cell, that’s enough for about 300,000 kilometers (186,000 miles).
What are the challenges and risks involved?
Hydrogen in gaseous form can be stored in tanks. However, in order to store a similar amount of energy as a gasoline fuel tank the tank would have to be as large as a warehouse hall. Alternatively, hydrogen can be liquefied at minus 253 degrees centigrade (–423.4 °F) in a very energy-intensive process. The solution is to compress the gas. Today’s hydrogen tanks are filled with a pressure of 700 bar (10,150 psi) – the inflation pressure of a normal tire is about 2 bar (29 psi). These hydrogen tanks have multi-layered walls made of diverse materials and are deemed to be crash-proof. Compared to gasoline and diesel fuel, hydrogen even has a safety advantage: it’s so volatile that an explosion is near-impossible. In tests run by the University of Miami, a car with a gasoline engine completely burned out whereas hydrogen burned in a huge flash within fractions of a second and then the fire went out
What components are needed to integrate fuel cells in vehicle powertrains?
The fuel cell itself is a rather compact component. In addition, the system includes air compressors and humidifiers, pumps, voltage controllers, the cooling and heating system, control electronics, a buffer battery and the hydrogen tank, which is the largest component, plus of course the electric traction motor.

How does hydrogen get to the filling station and into the fuel tank?
Special pressurized fuel trucks haul hydrogen to the filling station where it’s refilled into other pressurized tanks connected to the respective pumps. Filling hydrogen into a car’s fuel tank does not differ a lot from diesel or gasoline, except that there must be a pressure-resistant connection between the pump nozzle and the vehicle. This is ensured by a standardized system which is identical at all filling stations. Pipelines or liquid organic hydrogen carriers are alternatives to pressurized fuel trucks. In that case, hydrogen can be transported like any other fuel. At the filling station, the hydrogen is subsequently extracted from the carrier liquid again.

Where and when will it be used in the future?
Manufacturers like Nikola (USA) and Hyundai (South Korea) are planning to deliver the first truck tractors with fuel cell powertrains in 2020. German railroad operator Deutsche Bahn is currently testing trains with this technology in passenger transportation. They’re intended to replace conventional diesel locomotives on tracks without overhead wiring. In the aviation sector, manufacturers like Airbus and Boeing as well as research institutions such as the German Aerospace Center are intensively working on fuel cell aircraft. American manufacturer Zerovia is planning to equip at least smaller business aircraft with up to 20 passenger seats and ranges of up to 800 kilometers (500 miles) with fuel cell propulsion systems starting in 2022. Already close to production is the push-boat Elektra involving a consortium of partners including fuel cell manufacturer Ballard Power Systems. Fielding of the boat is scheduled to start in 2020 for hauling goods between Hamburg and Berlin on the rivers Elbe and Havel.
Where can fuel cells be used as energy sources for propulsion?
In all areas where battery-powered electric drive systems are either not economical or not feasible due to their size or weight and where long ranges with short refueling times are crucial: passenger cars, trucks, aircraft, ships and rail vehicles. “With fuel cells, they can also be operated with zero CO2 and other emissions,” says Professor Dr.-Ing. Tim Hosenfeldt, Senior Vice President Innovation and Central Technology at Schaeffler. The high “system efficiency of the fuel cell which clearly exceeds that of internal combustion engines primarily speaks for its use in means of transportation. In addition, local zero emissions of nitrogen oxide, CO2 and fine-dust particulate speak for this form of propulsion,” according to the German Federal Ministry of Transport and Digital Infrastructure.








